
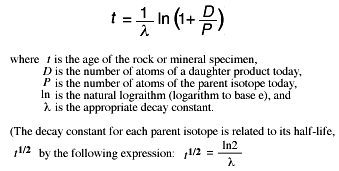

It's all about the simultaneous interactions among any three nucleons and the resulting influence on the decay of carbon-14. The reason involves the strong three-nucleon forces (a nucleon is either a neutron or a proton) within each carbon-14 nucleus. "And the underlying reason turned out to be a fairly exotic one." "This has been a very significant puzzle to nuclear physicists for several decades," said James Vary, an Iowa State University professor of physics and astronomy. Why, exactly, does carbon-14 have a half-life of nearly 6,000 years while other light atomic nuclei have half-lives of minutes or seconds? (Half-life is the time it takes for the nuclei in a sample to decay to half the original amount.) And while the carbon dating technique is well known and understood (the ratio of carbon-14 to other carbon isotopes is measured to determine the age of objects containing the remnants of any living thing), the reason for carbon-14's slow decay has not been understood.


 0 kommentar(er)
0 kommentar(er)
